Journal of Equine Veterinary Science –
Clinical Outcome of 14 Obese, Laminitic Horses Managed with the Same Rehabilitation Protocol (In Press)
www.hoofrehab.com/WhatsNew.html (link alla pagina di Pete Ramey)
www.sciencedirect.com/science/article/pii/S0737080613006370 (link diretto)
Questa documentazione di esperienza clinica espone, nello stile richiesto dalla comunità scientifica, quanto fatto da almeno 10 anni sul campo dai pareggiatori, “hoof care provider”, sia di Pete Ramey che di Jaime Jackson e da Jackson da più tempo ancora secondo protocolli diversi nella forma ma simili nella sostanza soprattutto per quanto riguarda la gestione, alimentazione, movimento controllato, protezione con accessori non permanentemente fissati allo zoccolo. Un successo senza pari. Qui i risultati sono documentati e elaborati statisticamente ai fini della pubblicazione del lavoro reso possibile dalla collaborazione tra la American Hoof Association e la Auburn University.
I principi sono espressi nel “Hoof Rehabilitation Protocol” della American Hoof Association di cui faccio parte come Member e Certified Trimmer. Lo trovate qui nel sito nella sezione AHA.
La metodica alla Auburn University é stata arricchita, per alcuni cavalli, con trattamenti farmacologici di sostegno che di sostegno appunto sono, acceleratori accettabili, condivisibili per il controllo del dolore, a seconda della circostanza e spesso non determinanti come l’esperienza precedente, più asciutta, sia mia che di Jaime Jackson e di altri, oltre naturalmente a quella di Pete Ramey, dimostra.
Trovate due casi di laminite cronica particolarmente indicativi risolti in tempi brevi nel periodo di una ricrescita dello zoccolo, sulla pagina “studio di zoccoli” di questo sito:
Cercate Brigitte e Asini Laminitici.
In questi due casi ho seguito lo stesso protocollo di riabilitazione.
Se volete un commento e una esposizione della tecnica impiegata da Taylor-Ramey e una panoramica di quanto é possibile fare e ottenere comunque senza l’ausilio della diagnostica per immagini sono disposto a raggiungervi nella sede che riterremo più opportuna.
Dr. Franco Belmonte
Biologo, AHACT
Clinical Outcome of 14 Obese, Laminitic Horses Managed with the Same Rehabilitation Protocol
Debra Taylor DVM, MS,
Alex Sperandeo, John Schumacher DVM, MS,
Thomas Passler DVM, PhD,
AnneWooldridge DVM, PhD,
Rhodes Bell DVM,
Adam Cooner DVM,
Leah Guidry BS,
Hannah Matz-Creel DVM,
Ivy Ramey,
Pete Ramey
ABSTRACT
A specific method of rehabilitation was used to manage obese horses with laminitis, and clinical outcome was evaluated after 5 to 20 months. Clinical data from 14 similar laminitis cases were statistically analyzed to evaluate response to rehabilitation. Data were analyzed using repeated measures or logistic regression methodologies. Each horse presented as obese and laminitic with no history of a systemic inflammatory disease. The rehabilitation method emphasized a mineral-balanced, low nonstructural carbohydrate diet; daily exercise; hoof trimming that minimized hoof wall loading; and sole protection in the form of rubber hoof boots and/or hoof casts. Distal phalanx alignment within the hoof capsule was significantly improved, and hoof wall thickness was significantly decreased (P <.0001) following treatment. Solar depth was significantly increased (P < .0015). Reduction of palmar angle measurements was detected in acutely and chronically affected horses. This treatment effect was statistically greater for horses with chronic laminitis than for horses with acute laminitis (P interaction < .0001). Horses were 5.5 times more likely to be sound post-treatment than before treatment. Daily exercise, dietary modification, and removal of ground reaction force from the hoof wall were foci of the rehabilitation program. Hoof care and husbandry as applied to these horses may be an effective method of rehabilitation of horses from obesity-associated laminitis.
1. Introduction
It is generally accepted that prognosis for laminitic horses with significant palmar rotation of the distal phalanx is guarded to poor [1]. Other indicators predicting outcome of laminitic horses include severity of lameness [2], white blood cell count [3], weight of the horse [4], number of feet involved [5], and the magnitude of the corrected (for magnification) distance between the proximal aspect of the extensor process of the third phalanx and the most proximal extent of the proximodorsal wall measured on a lateromedial radiograph [6]. The inciting cause of laminitis may also be a factor in outcome. Recent studies indicate that the basement membrane of epidermal laminae of ponies with insulin-induced laminitis remains intact [7] and minimal upregulation of matrix metalloproteases occurs in the laminae when laminitis is insulin induced [8]. Preservation of the basement membrane in these laminitic ponies suggests that some horses with endocrinopathic laminitis mayhave complete or at least improved hoof repair once the ongoing insulin-induced laminar insult is eliminated.
Results of studies indicate that exercise and controlled feed intake decrease insulin resistance in ponies [9-11]. However, exercising laminitic horses is controversial when movement causes pain and/or may further damage inflamed lamina.
The goal of this study was to evaluate the outcome of laminitic horses subjected to a specific management program that emphasized a mineral-balanced, low nonstructural carbohydrate diet; daily exercise; hoof trimming that minimized hoof wall loading and reestablished a lower palmar angle; and sole protection in the form of rubber hoof boots and/or hoof casts (see sections 2.3 to 2.7 below) when needed for horse comfort and safety. This program was expected to improve foot morphology, radiographic parameters, and gait. The medical records of 14 obese, laminitic horses that participated in the management program were examined, and objective parameters were statistically analyzed.
2. Materials and Methods
2.1. Case Selection
The medical records of 14, obese (body condition score >6), laminitic horses that had acutely or insidiously developed bilateral forelimb (13 horses) or bilateral forelimb and hindlimb (1 horse) lameness with clinical and radiographic signs of laminitis were evaluated. A horse’s medical record was included for evaluation if the horse had a history of each of the following: development of laminitis while on grass pasture, a presenting Obel lameness score >=2 [12] (subjectively determined by D.R.T.), radiographic evidence of palmar rotation of the distal phalanx or proximal rotation of the hoof capsule of both forelimbs, a presenting body condition score >6, and management with the protocol described below. Horses that had either divergent growth rings of the hoof capsule or remodelling of the distal phalanx, or both, were classified as having “chronic” laminitis, and those who lacked divergent growth rings of the hoof capsule and had no evidence of remodelling of the distal phalanx at the time of presentation were considered to have acute laminitis. “Lipping” of the distal phalanx on the lateral radiograph was considered evidence of remodelling. Growth rings that were wider in the heel region than in the toe region were considered divergent. Statistical analysis was performed using clinical data at 2 time points: initial presentation and when the client requested the final radiographic reevaluation and the horse had returned to its previous level of soundness (5-12 months postinitial presentation).
2.2. Radiographs and Measurements
All lateromedial radiographs of the front feet were acquired by the same clinician (D.R.T.) [13], using criteria described by Redden [14,15], and included the following recommended standards: a true lateral projection with the primary beam striking the foot in a horizontal plane 1 cm above the bearing surface; a zero subject-to-film distance by ensuring that the medial aspect of the hoof was in contact with the radiographic cassette and maintaining a consistent distance between the radiograph machine and the cassette; detailing the face of the hoof wall with barium; and having the horse standing on 2 wireembedded positioning blocks of equal height, with the limbs in a vertical position. The proximal aspect of the hoof capsule and dorsal margin of the hoof capsule in all cases had been marked with barium paste to bisect the foot from the roots of the most distal hairs of the coronary band to the tip of the hoof capsule at the bearing surface. The sagittal plane of the sole of the hoof was marked with barium on some horses.
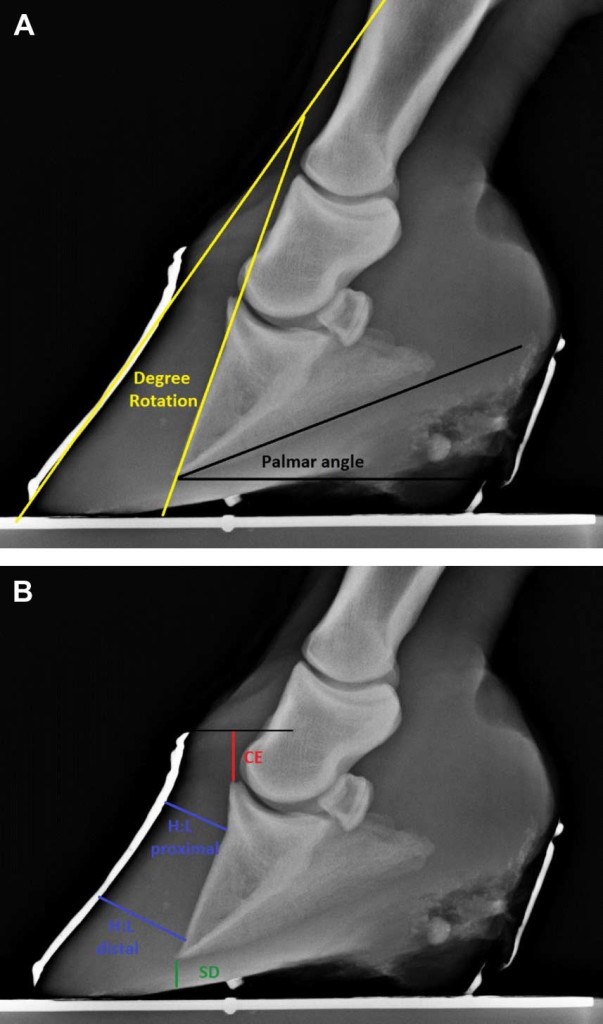
Five radiographic parameters were measured and recorded for statistical analysis (Fig. 1A, B): (1) The thicknesses of the dorsal horn and lamellar tissues were measured proximally at the level of the base of the extensor process and distally at the level of the tip of the distal phalanx (or just proximal to “lipping” if present).
Fig. 1. (A) Method used to measure the degree of rotation (yellow angle) and the palmar angle (black angle), which were evaluated before and after treatment on both front feet of each horse. (B) The red line shows the measurement of the horizontal distance from the coronary band to the extensor process (CE); the green line shows sole depth measurement (SD), and the blue lines show measurement locations of the thickness of the proximal and distal aspects of the dorsal hoof wall (H:L zones). Each of these measurements was evaluated before and after treatment on both front feet of each horse.
These proximal and distal measurements of dorsal hoof wall thickness were made on lines drawn perpendicular from the dorsal cortex of the distal phalanx to the edge of the barium marker that had been applied to the dorsal hoof wall. The numerical difference between the proximal and distal measurements (distal measurement – proximal measurement ¼ numerical difference between thickness of proximal and distal dorsal hoof wall) was used for statistical analysis purposes as an indicator of the relationship of the dorsal hoof wall to the dorsal cortex of the distal phalanx. (2) Solar depth was measured on a perpendicular line drawn distally from the tip of the third phalanx to the outer border of the sole or the proximal margin of the barium column (if the mean sagittal plane of the sole had been outlined with barium). (3) The palmar angle was measured as the angle of incidence between the solar margin of the distal phalanx and the bearing surface. (4) The vertical distance from the horizontal plane through the proximal extent of the hoof capsule to the most proximal aspect of the extensor process of the distal phalanx was measured and described as the coronary band: extensor process distance [15], previously designated the founder distance [6]. (5) The degrees of rotation between the dorsal hoof wall and the dorsal cortex of the distal phalanx were measured.
Raw radiographic measurements were used for statistical analysis without correction for innate magnification because a zero subject-to-film distance was always used, and absolute numbers were compared only to previous radiographic measurements determined for the same horse.
2.3. Management Protocol: Hoof Care
The hooves of all horses were routinely trimmed (every 3 or every 6 weeks by P.R. or A.S.). The basic concept of the trim strategy was to eliminate weight bearing by the hoof walls by promoting weight bearing by the sole, bars, and heel buttress and to prevent increasing elevation of the palmar angle created by excessive heel growth relative to toe growth. The length of the trim cycle, 3 versus 6 weeks, was based on each horse’s current heel-to-toe hoof growth pattern. Heels were lowered during each trim based on the following parameter: the heel plane was lowered to 0.25 inch above the live sole without removing more than 10 mm of heel at any one trim. Therefore, horses that were growing much more hoofwall in the heel region than in the toe region required a 3-week trim cycle during the first few months of treatment. The trim cycle was lengthened to 6 weeks as soon as the heel-to-toe hoof growth ratio had equalized sufficiently so that the heel height did not vary by more than 10 mm during that time period [16].
Fig. 2. (A) The basic shape of the trim is shown. Note the hoof wall is trimmed to the level of the sole plane and then bevelled to minimize weight bearing by the hoof wall at the toe and through the quarters. (B, C) Hooves from 2 different horses are shown during a routine trim. Note that a heel plane is being established by the rasp on each hoof. This plane is approximately 2° to 3° away from the palmar angle (solar plane of the distal phalanx).
At the time of each trim, the hoof walls were trimmed to a length level with the sole and then bevelled to minimize weight bearing by the hoof walls (Fig. 2A). Thin soles were protected as described below to maximize horse comfort and safety. Hoof wall flares were not rasped on the outer surface of the hoof until the new growth of the hoof wall had grown at least two-thirds of the distance from the hairline to the ground. During each trim, a heel plane that was approximately 2° to 3° degrees positive to the palmar angle and 0.25 inch above the live sole plane was established (Fig. 2B).
After trimming so that the sole, rather than the wall, bore weight, solar pressure was minimized by using one or more of the following methods of solar protection: (a) application of removable strap-on hoof boots1,2 (Fig. 3) with foam rubber pads1,3 and/or dental impression material4,5; (b) application of hoof casts6 to cover pads1,3 and/or dental impression material4,5 that had been applied to fill the solar concavity and collateral sulci; (c) application of glue-on hoof boots1 with dental impression material4,5 filling the solar concavity and collateral sulci; or (d) allowing the horse to go barefoot on yielding terrain including soft ground free of rocks or loose beds of pea gravel (5- to 8-mm-diameter stones) 10 cm deep.
Fig. 3. Two styles of protective hoof boots1,2 used to protect and hold soft protective pads1,3 in place on the feet of the horses of this
Removable, strap-on hoof boots1,2 with pads1,3 were always the first choice as an initial method of solar protection and were used as needed for horse comfort and safety on the available terrain. Horses wore hoof boots nearly 24 hours per day until their use no longer improved the level of horse comfort. Horse comfort was subjectively judged based on the Obel lameness scale and the horse’s daily habits of self- initiated movement and recumbency. Owners were instructed to remove the strap-on hoof boots with pads from the hooves briefly each day to allow cleaning and drying of the hoof and the boot. During the later stages of treatment (when the horse had 2-3 cm of uniform new hoof growth from the coronary band), removable hoof boots (used as a method of solar protection) were, for some horses, replaced by hoof casts or glueon hoof boots (for periods of 6-12 weeks). The decision to use hoof casts or glue-on hoof boots (in lieu of removable strap-on boots) was based on one of following factors: the owner’s inability to maintain the removable strap-on hoof boots or the need for establishment of a shallow (5- to 6-mm) air space under a very thin sole (ie, <7 mm) or a solar perforation or defect. Hoof casts were always chosen over glue-on boots when the need to establish and secure pads with cutout air spaces under jeopardized sole regions arose. The use of deep pea gravel footing for horses with sole depths of 8 to 10 mm or more was encouraged. It was noted that rock diameter needed to be matched to sole depth in order to observe signs of increased comfort. Use of pea gravel footing was recommended only for periods of rehabilitation process when the current sole depth and pea gravel diameter combination allowed horses to exhibit signs of increased comfort while on the gravel. Signs of increased comfort included (1) the horse choosing to stand in the gravel rather than other terrain, (2) the horse turning more easily in the gravel, (3) the horse spending more time standing while on the gravel (as opposed to other available terrain), and (4) the horse assuming a more normal stance when standing on the gravel. Barefoot turnout was not allowed until a horse had sole depth >=12 mm and was able to move comfortably on the current terrain without hoof protection.
2.4. Management Protocol: Diet
The weight loss goal was to decrease each horse’s body condition score to a five [18]. Dietary restrictions recommended for each laminitic horse included elimination of grains and/or processed feeds, elimination of fruits, vegetables, and other sweet or starchy treats, partial or complete restriction from pasture grazing (this restriction varied according body condition of the horse and size or condition of available pasture). Horses were fed Bermuda grass hay ad libitum. During the initial weight loss period, owners were advised to have their hay tested for nutrient content. Advice was also offered at the onset of treatment that any untested hay be soaked inwater prior to feeding in effort to minimize the chance of feeding high nonstructural carbohydrate hay. Retrospectively, none of the owners reported having tested the types of hay fed to these 14 horses. Owners reported soaking their hay in water for various time periods ranging from 30 minutes to 12 hours prior to feeding. Mineral supplementation was provided empirically to balance suspected nutritional content of local hay/ grass to meet recommendations of the National Research Council [17].
2.5. Management Protocol: Exercise
Turnout in a grass-free paddock or daily in-hand exercise was encouraged after the following (1) hooves had been trimmed to minimizeweight bearing by the hoofwall; the desired heel plane and palmar angle (<= 10 degrees) had been established by trimming; and when the hooves had been protected by soft protective hoof boots with pads (as described in Section 2.3) that provided enough comfort for the horse to have a heel-first hoof landing. In-hand exercise was increased daily by adding 5- to 10-minute increments to each exercise session until horses were walking 30 to 45 minutes 2 or 3 times daily. Owners were instructed to observe for the intended heel-first impact of the hoof boot and to walk the horse only while the hoof boots were securely in place. They were instructed not to walk the horse if the hoof impact appeared to be toe-first and to discontinue daily walking (and call the veterinary/ hoof care provider team) if the horse seemed to have increasing pain after walking.
2.6. Management Protocol: Medication
Phenylbutazone (4.4 mg/kg once every 24 hours or 2.2 mg/kg once every 12 hours orally) was used for ameliorating pain when needed to maintain an Obel grade score <=2. Acepromazine (20 mg every 8 hours intramuscular) was administered to 6 horses as a peripheral vasodilator during the initial 2 to 4 weeks of therapy. The soles of horses with solar necrosis and prolapsed solar corium (5 horses) were treated with topical tetracycline and/or metronidazole. One horse with solar necrosis received intravenous oxytetracycline (7 mg/kg every 12 hours intravenously for 14 days). During periods of active abscess formation and/or drainage of exudates from the sole, owners were advised to soak the feet 3 to 5 times weekly in either magnesium sulfate solution or 50% acetic acid solution.
2.7. Body Condition Scoring
The body condition scores of horses were determined by D.R.T., using the system described by Henneke [18] before treatment and at the endpoint radiographic examination after the owners reported a return to the prelaminitis level of soundness.
2.8. Statistical Analysis
Response variables, including coronary band-toextensor process distance, palmar angle, degree of rotation, sole depth, and difference of the dorsal hoof wall thickness at the proximal and distal aspects, were measured or scored before and after treatment, hence the data had the character of repeated measures, ie, multiple observations on the same experimental unit. Data were analyzed using repeated measures methodology as implemented in SAS(R) PROC GLIMMIX.7 To account for the possibility of an interaction, data were analyzed using repeated measures methodology using a linear model in which the main effects condition (condition refers to chronicity (acute or chronic) of laminitis) and treatment as well as their interaction were included. Inspection of studentized residuals (¼ standardized residuals distributed as a t random variable) indicated that the normally assumption was warranted for all response variables, except OBEL. The interaction was significant only for palmar angle (P ¼ 0.002). Because all other response variables had a nonsignificant interaction term (P >= 0.63) it was dropped from the model resulting in a main effects model. Significance of pairwise differences was calculated using the PDIFF or SLICEDIFF option of the LSMEANS statement in the above named PROC. The dichotomous OBEL before and after treatment scores were analyzed using logistic regression as implemented in in SAS(R) PROC LOGISTIC. Means and 95% confidence intervals on the logit scale were back-transformed to frequencies.
2.9. Long-term Follow-up Owner Survey
Owners of 12 horses were contacted 23 to 73 months after initial onset and asked the following questions: “Has the horse had any foot pain due to laminitis relapses or hoof abscesses since the follow-up radiographic exam?” and “Is your horse currently considered rideable?”
3. Results
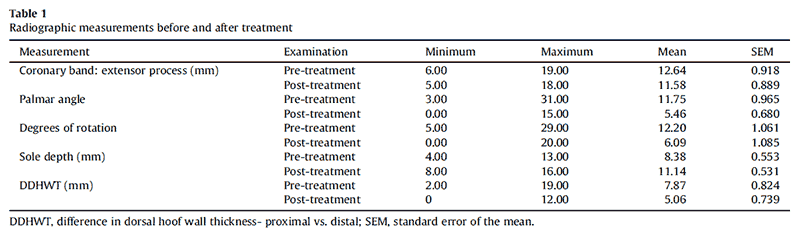
Six different breeds were represented within this group of horses (7 Tennessee Walking Horses, 2 Appaloosa, 2 Quarter Horses, 1 grade horse, 1 Rocky Mountain Saddle Horse, and 1 Peruvian Paso; 10 geldings and 4 mares, ranging in age from 4-22 years; mean age, 13 years). Descriptive information for the measurements taken at pre- and post-treatment examinations is given in Table 1. At initial examinations, the median Obel lameness score for all pretreatment laminitic horses was 3.5 (range: 0.5-4). In contrast, at post-treatment evaluations, 12 horses appeared subjectively to be sound at a trot or equivalent gait, and the median Obel lameness score for all horses was 0 (range: 0- 1). All owners considered the quality of their horse’s gait to be equivalent to their prelaminitic gait. One of the authors (D.R.T.) evaluated the gaits of all horses at the time of endpoint radiographic examination and subjectively scored 12 horses (0 of 10) and 2 horses appeared mildly lame at a trot (2 of 10). Statistically, horses were 5.5 times more likely to be sound at post-treatment examination than at pre-treatment examinations. A statistically significant decrease of the third phalanx rotation was detected posttreatment (P < .0001, 95% CI for differences: 3.8°-6.5°). The differences of the dorsal hoofwall thicknesses between the proximal and distal aspects of the distal phalanx was significantly reduced at post-treatment evaluation (P < .0001, 95% CI for differences: 2.0-3.5 mm). The reduction of the coronary band-to-extensor process distance was not statistically significant (P ¼.10, 95% CI for differences: -0.2- 2.3 mm). Solar depth was significantly increased at posttreatment evaluation (P ¼ .0015, 95% CI for differences: 3.1-5.4 mm). An overall reduction of palmar angle measurements was detected in acutely and chronically affected horses. This treatment effect was statistically greater for horses with chronic laminitis than for horses with acute laminitis (P interaction < .0001).
A decrease of the body condition scores of each horse was observed during the treatment period, and while the median body condition score was 8.5 at initial evaluation, a median body condition score of 5 was observed at posttreatment. One horse became obese again subsequent to pasture turn-out in the spring–summer season posttreatment, but, according to the owner, primary care veterinarian (D.R.T.) and hoof care professional (A.S.), remained sound while obese.
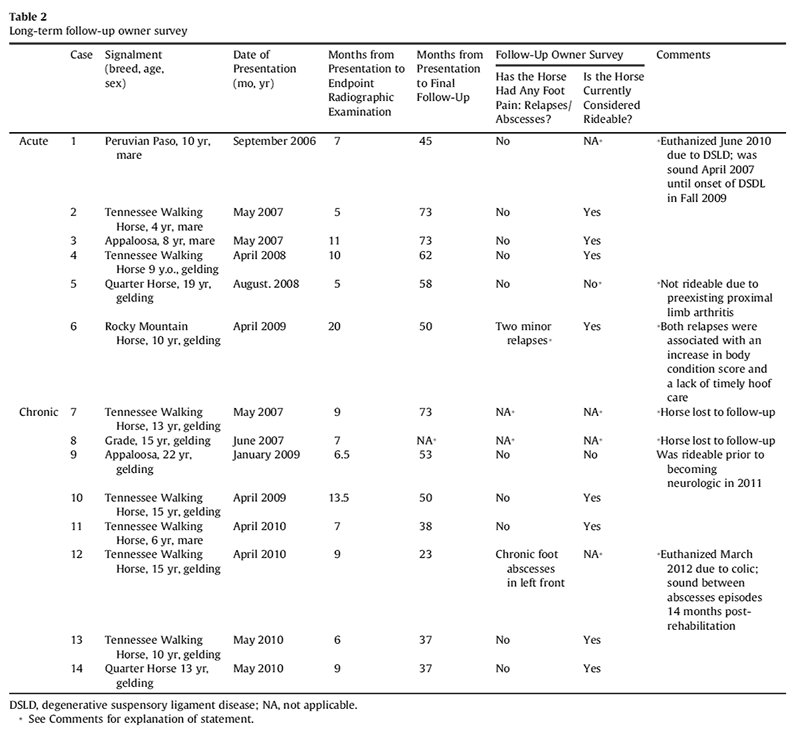
Results of long-term follow-up (2-6 years after initial onset of laminitis) of 12 horses indicated that 8 of 12 owners considered their horse to be rideable (2 horses were lost to follow-up and 2 horses were euthanized due to nonlaminitis-related diseases; 1 acquired chronic neurologic deficits, and 1 has pre-existing arthritis of the proximal pelvic limbs). Only 1 owner reported transient foot pain due to apparent laminitis relapse after allowing the horse unlimited access to pasture, resulting in body condition score of 8 in conjunction with a lapse in hoof care. One other owner reported recurrent abscess episodes in one foot (Table 2).
4. Discussion
It is likely that all horses in this study suffered from endocrinopathic (grass-induced) laminitis because each was obese and there was no history or clinical evidence of an acute systemic inflammatory disease or clinical signs of pituitary disease. It is possible that some of these horses suffered from pituitary pars intermedia dysfunction, but the horses had no clinical signs of this syndrome before or after the study. Because of the retrospective nature of this study, insulin concentrations were determined only for a few horses, and other measures of glucose homeostasis were not performed in any of the horses; therefore, the data were not analyzed.
The prognosis for return to former athletic function for horses with >11.5° of distal phalangeal rotation is considered poor, and the prognosis for horses with 5.5° to 11.5° of rotation is guarded [1]. Even though all horses in this study had >5.5° rotation of the distal phalanx and 6 had >11.5° of rotation, all returned to their prelaminitis level of soundness as evaluated by owners. While it would have been ideal for a blinded unbiased clinician to have performed lameness evaluation on each of these horses, they were managed as field cases by a single veterinarian (D.R.T.), and there was no clinical indication for objective evaluation of gait at the time of initial and subsequent evaluations.
After laminar failure, the primary mechanical force working to separate the laminae is the weight of the horse opposed by the hoof wall [19]. When the hoof wall bears weight, the lamellae are forced to suspend the horse’s weight and bear foot impact forces. Even though tension of the deep digital flexor muscle and tendon exerts a rotational force on the distal phalanx, we suspect that tension of the deep digital flexor tendon did not result in added stress to laminae in these horses because the hoof wall in the toe region was trimmed so it remained out of contact with the bearing surface.
Because a significant overall reduction of the palmar angle of these horses was detected (from a pretreatment mean of 11.75° to a post-treatment mean of 5.46°), this method of laminitis management should be further investigated as an alternative nonsurgical method for restoring alignment of the distal phalanx [20] in laminitic horses.
The treatment effect on palmar angle reduction was statistically larger for chronic cases than for acute cases.
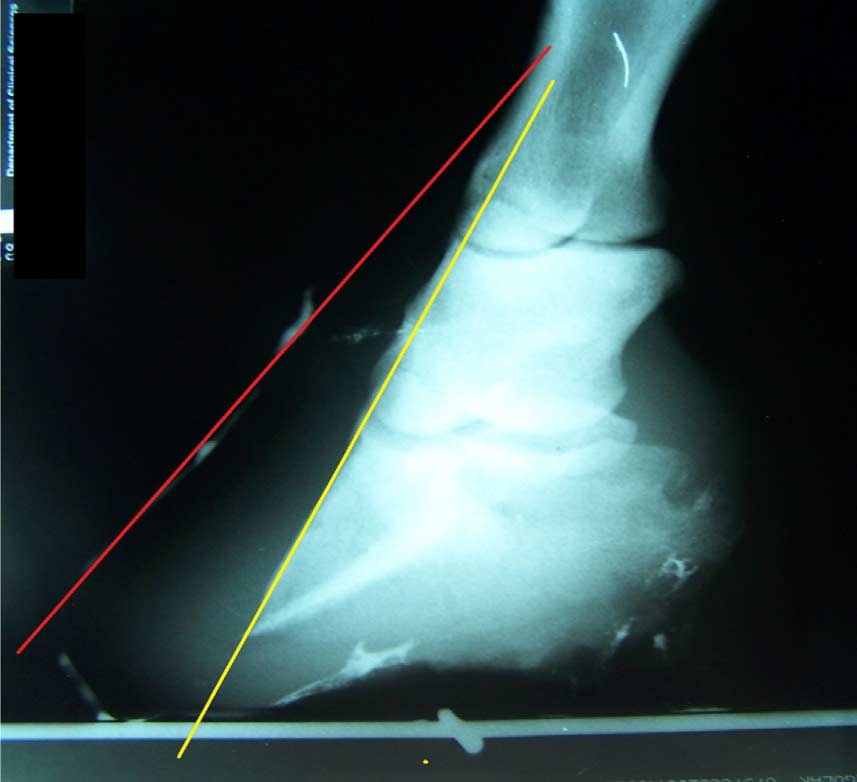
Fig. 4. Oblique radiographic view of the left front foot of one of the subject horses. Note that the red line, which represents the hoof wall, is not parallel to the yellow line, which represents the cortical surface of the distal phalanx. This radiograph provides evidence that obesity-associated laminitis may produce lamellar pathology resulting in wall flares in the quarters as well as in the toe regions [16].
The authors believe that the amount of lamellar separation at the quarter walls (or anywhere around the perimeter of the foot) may be as significant as the amount of lamellar separation in the region of the toe [16] (Fig. 4). Eliminating or at least reducing weight-bearing by the entire perimeter of the hoof wall (with the exception of the heel buttress) may be important in stopping and possibly reversing distal decent of the third phalanx in horses with either acute or chronic laminitis. Reduction or elimination of weight-bearing by the hoof wall theoretically decreases strain on laminar attachments by removing mechanical forces, thus minimizing or preventing additional rotation or sinking [21]. Elimination of weight-bearing by the hoof wall may also reverse distal descent of the distal phalanx in some horses as demonstrated in lateromedial radiographs of the right front foot of horse 11 (Fig. 5) obtained at initial evaluation and 2 years 7 months later.
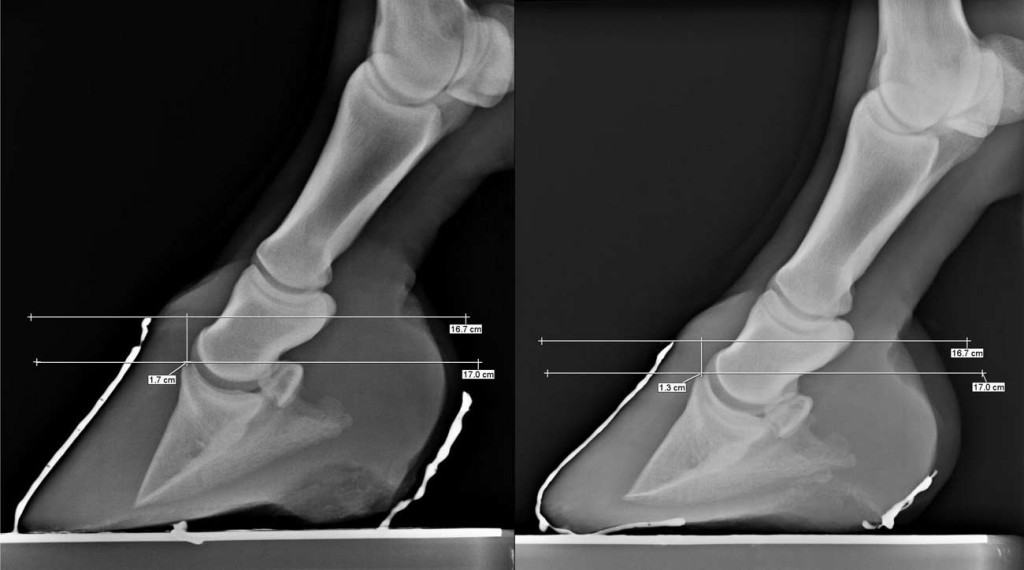
Fig. 5. The radiographs8 show that the coronary band to extensor process distance (CE) may become smaller over time in this hoof care system. The radiograph at right (CE ¼ 13 mm) is a follow-up examination of the right front foot 2 years and 7 months after the initial evaluation in the radiograph on the left (CE ¼ 17 mm).
This hoof care method used the sole to support the distal phalanx. While using the sole to support the distal phalanx, it is important that the sole be protected during weight-bearing yet not be subjected to pressure during hoof flight. When sole thickness is <12 mm and/or when weight-bearing by the hoof wall is eliminated, excess pressure on the solar corium can result. When the sole under the margin of the distal phalanx was less than 7 mm, bulging, or cracked, an air space had been established in the hoof pad under the solar margin. We suspect that lack of solar pressure during hoof flight is critical to maintaining solar blood flow to prevent solar corium injury. Each method of solar protection used in these cases was intended to release pressure on the solar corium during hoof flight. Efforts were made to protect the laminar corium by allowing the hoof wall to bear little or no weight by beveling the hoof wall away from the ground and not allowing application of a shoe to the hoof wall (Fig. 2).
When the horse’s foot is bearing weight, arterial blood flow to the foot is occluded, in part, by force of the deep digital flexor tendon on the medial and lateral digital arteries. This is a normal physiological event, and it is the likely mechanism preventing backflow of arterial blood during the loading phase of the stride [22]. When the foot does not bear weight, arterial blood flow to the foot is restored. We suspect that exercise of these horses caused an intermittent lack of pressure to the sole and prevented ischemia of the laminar and solar corium that occurs when the horse’s foot bears weight continuously. It is important to understand that these horses were exercised with the hoof wall bevelled to minimize weight-bearing by the hoof wall, the sole well protected by soft pads held on the hoof by hoof boots or occasionally casts and a near normal palmar angle of less than 10°. If horses did not land heelfirst in their protective hoof wear during exercise, exercise was discontinued until this desired hoof landing was achieved by altering the hoof mechanics and padding [16]. The exercise regimen seemed to alleviate pain in these horses as shown by increased comfort (decreased pain – modified Obel pain scale) [22] during and after exercise. Exercise is recommended for horses suffering from insulin sensitivity [9,11]. This laminitis management method may provide a means of exercising insulin resistant laminitic horses without jeopardizing their hooves. These horses were fed a diet that was intended to minimize the intake of nonstructural carbohydrates, provided free-choice access to hay and also provide a mineral-balanced diet.
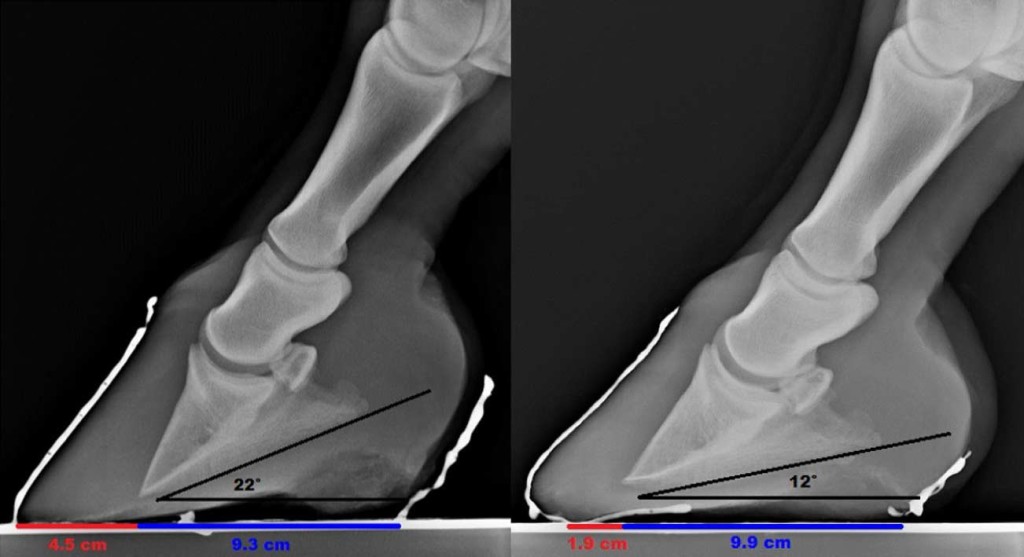
Fig. 6. Radiographs8 show change in the dimension of the soft tissues of the heel and shortening of the digital breakover over 2 years 6 months (left image, April 2, 2009; right image, October 20, 2011). Note that the weight-bearing surface of the heel region has increased 6 mm in length (from 9.3 to 9.9 cm). Also note the realignment of the bones of the distal limb and the lowering of the palmar angle (pre: 22°; post: 12°).
Because none of the types of hay fed to the 14 horses of this study were tested for nutritional content and owners soaked the hay for various amounts of time, the actual dietary intake of these horses could not be determined retrospectively. The hay “as fed” combined with the exercise program “as applied” did cause a statistically significant decrease in body condition score, which indicates that the diet and exercise regime was clinically appropriate. However, close attention to diet to include nutrient testing of hay fed to obese laminitic horses would obviously be superior to the empirical feeding programs of these horses.
This method of laminitis management apparently alleviated pain and returned this group of horses to their former level of athletic ability despite suspected minor to moderate nutritional imbalance, distal phalanx remodelling in some horses, and incomplete resolution of hoof wall rotation in some horses (Fig. 5). It is hypothesized that increased heel volume (Fig. 6) may in some way compensate for distal phalanx remodelling and laminar damage as altered heel dimension similar to the improved heel angles described by Clayton et al [23] was also noted in these horses.
As none of these horses was hospitalized during the laminitis treatment period, owner compliance with daily care recommendations likely played a role in the favorable outcome in these horses. Successful management of obese, laminitic horses requires a concentrated long-term team effort among the veterinarian, the owner, and the hoof care professional to manage the horse’s diet, exercise, hoof care, and medical needs. In this series of cases, the radiographic parameters of the hoof improved, and lameness decreased during the period of dietary restriction, weight loss, and exercise.
Using the described management protocol, 14 of 14 laminitic horses with rotation >=5° and a guarded prognosis returned to their prelaminitis level of soundness by the time of the endpoint radiographic evaluation. Long-term follow-up survey indicated that most of these horses maintained the same level of soundness without incidence of laminitis recurrence or hoof abscess formation. This method of laminitis management is effective and warrants further evaluation.
Acknowledgments
We would like to acknowledge the dedicated owners and care takers of the horses described in the manuscript.
References
[1] Stick JA, Jann HW, Scott EA, Robinson NE. Pedal bone rotation as a prognostic sign in laminitis of horses. J Am Vet Med Assoc 1982; 180:251–3.
[2] Hunt RJ. A retrospective evaluation of laminitis in horses. Equine Vet J 1993;25:61–4.
[3] Coffman JR, Hammond LS, Garner HE, Thawley DG, Selby LA. Haematology as an aid to prognosis of chronic laminitis. Equine Vet J 1980;12:30–1.
[4] Coffman JR, Johnson JH, Finocchio EJ, Guffy MM. Biomechanics of pedal rotation in equine laminitis. J Vet Med Assoc 1970;156: 219–21.
[5] Colles CM, Jeffcott LB. Laminitis in the horse. Vet Rec 1977;100: 262–4.
[6] Cripps PJ, Eustace RA. Factors involved in the prognosis of equine laminitis in the UK. Equine Vet J 1999;31:433–42.
[7] Asplin KE, Patterson-Kane JC, Sillence MN, Pollitt CC, McGowan CM. Histopathology of insulin-induced laminitis in ponies. Equine Vet J 2010;42:700–6.
[8] de Laat MA, Kyaw-Tanner MT, Nourian AR, McGowan CM, Sillence MN, Pollitt CC. The developmental and acute phases of insulin-induced laminitis involve minimal metalloproteinase activity. Vet Immunol Immunopathol 2011;120:275–81.
[9] Freestone JF, Beadle R, Shoemaker K, Bessin RT, Wolfsheimer KJ, Church C. Improved insulin sensitivity in hyperinsulinaemic ponies through physical conditioning and controlled feed intake. Equine Vet J 1992;24:187–90.
[10] Stewart-Hunt L, Geor RJ, McCutcheon LJ. Effects of short-term training on insulin sensitivity and skeletal muscle glucose metabolism in Standardbred horses. Equine Vet J Suppl 2006;36: 226–32.
[11] Frank N, Geor RJ, Baily SR. Equine metabolic syndrome. ACVIM consensus. J Vet Intern Med 2010;24:467–75.
[12] Obel N. Studies of the Histopathology of Acute Laminitis [dissertation]. Uppsala, Sweden: Almqvist and Wilksells Boktryckeri AB; 1948.
[13] Taylor DR. Radiographic imaging of the laminitis patient. In: Ramey P, editor. Care and rehabilitation of the equine foot. Lakemont, GA: Hoof Rehabilitation Publishing LLC; 2011. p. 234–63.
[14] Redden RF. Shoeing the laminitic horse. In: Norwood G, editor. Proceedings of the Forty-third Annual Convention of the American Association of Equine Practitioners. Phoenix, Arizona, USA: AAEP Publishing; 1997. p. 356–9.
[15] Redden RF. Clinical and radiographic examination of the equine foot. In: Bramlage L, editor. Proceedings of the Forty-ninth Annual Convention of the American Association of Equine Practitioners. New Orleans, Lousianna, USA: AAEP Publishing; 2003. p. 169–85.
[16] Ramey P, editor. Care and rehabilitation of the equine foot. Lakemont, GA: Hoof Rehabilitation Publishing LLC; 2011. p. 284–352.
[17] National Research Council nutrient requirements of horses, 6th rev. ed. Washington, DC: National Academies Press; 2007.
[18] Henneke DR. A condition score system for horses. Equine Pract 1985;8:13–5.
[19] Steven D. Functional anatomy of the horse’s foot. Pract 1981;3:22–7.
[20] O’Grady SE. How to restore alignment of P3 in horses with chronic laminitis. In: Bramlage L, editor. Proceedings of the Fortyninth Annual Convention of the American Association of Equine Practitioners. New Orleans, Lousianna, USA: AAEP Publishing; 2003. p. 328–36.
[21] Ramey P. Care and rehabilitation of the hoof walls and lamellar attachment. In: Ramey P, editor. Care and rehabilitation of the equine foot. Lakemont, GA: Hoof Rehabilitation Publishing LLC; 2011. p. 345.
[22] Van Eps A, Collins SN, Pollitt CC. Supporting limb laminitis. Advances in laminitis. Part II. Equine Pract 2010;26:287–302.
[23] Clayton HM, Gray S, Kaiser LJ, Bowker RM. Effect of barefoot trimming on hoof morphology. Aust Vet J 2011;89:305–11.